Science is all about order, and to keep it simple, we have thousands of different terminologies. Sometimes molecule vs. compound gets confusing; that’s why I have chosen this topic to elaborate on the difference between molecule and compound.
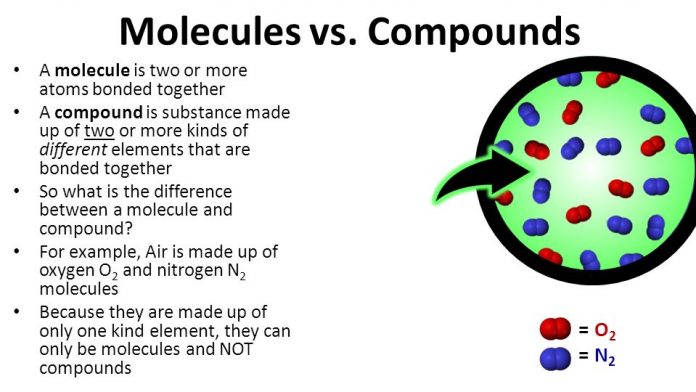
Compounds and molecules are related to each other because all compounds are molecules. However, the difference between molecule and compound is not all molecules are compounds.
To understand molecules and compounds, it is important to know about the atom.
Atom is a particle of matter that defines a chemical element. An atom consists of a nucleus of positively charged protons, electrically neutral neutrons, and a negatively charged electron. Molecules and compounds are made up of atoms chemically bonded together through ionic bonding, covalent bonding, and metallic bonding.
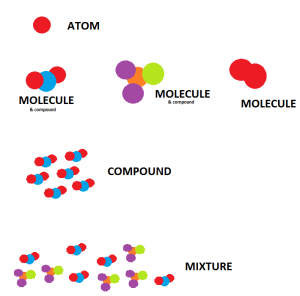
Molecules:
A Molecule is a particle or a substance made up of two or more atoms of the same or different groups of chemically bonded atoms.
Molecules may be simple or complex. Some common examples of molecules are
- Water (H2O)
- Nitrogen (N2)
- Ozone (O3)
- Calcium oxide (CaO)
- Glucose (a type of sugar) C6H12O6
- Sodium chloride (table salt) NaCl
- Hydrochloric acid (HCL)
Single atoms are not molecules such as oxygen (O2), Hydrogen (H2).
There are three types of molecules such as element molecule, compound molecule, and mixture molecule.
Element molecule: Difference between molecule and compound
The element molecules are made up of similar atoms that combine. The element molecule formed of one atom call monatomic molecules such as solid (iron, copper, sulfur, and carbon), liquid (mercury), and noble gasses like (helium, neon, and radon).
Element molecules formed of two atoms are diatomic molecules, such as liquid (bromine) and active gases (oxygen, hydrogen, and nitrogen). Molecule of active gasses are diatomic, and molecules of inert- gasses are monoatomic.
Compound molecule
The compound molecule comprises different atoms, such as sodium chloride NaCl and ammonia molecule. Compound molecules can break down into simpler atoms chemically, not physically.
Mixture molecule
The mixture molecule is made up of two or more different compounds that are physically united. These molecules can separate into their components by physical means.
Compounds
Most individuals find it hard when they are asked to describe a compound. Many of them get confused about the terms and start explaining molecules instead of compounds. Therefore, it is important to explain what a compound is. In most simple words, a compound is a mixture of two or more two elements that chemically attach.
Example of Compound
There are hundreds of examples available that can use to explain compounds, but we prefer the example from daily life. For instance, almost every one of us has some idea about carbon dioxide. The formula of carbon dioxide is CO2, which clearly explains that this is a mixture of carbon and oxygen.
At last
Molecules and compounds are two of the most used terms. Especially in the educational and chemical labs. The molecule is a combination of two atoms. On the other hand. A compound is a combination of two or more elements. The mentioned above information will help you in understanding. The difference between both the terms comprehensively.
Read Also: how to clean a fish tank
hello there and thank you for your info – I have definitely picked up anything new from right here.
I did however expertise some technical issues using this site, as I experienced to
reload the website lots of times previous to I could
get it to load correctly. I had been wondering if your web host is OK?
Not that I’m complaining, but slow loading instances times will very frequently affect your placement in google and can damage your quality score if advertising and marketing with Adwords.
Anyway I’m adding this RSS to my e-mail and could look out for much more of your respective interesting content.
Make sure you update this again soon.
Can provide a link mass to your website https://ztd.bardou.online/adm
Your site’s position in the search results https://ztd.bardou.online/adm
Free analysis of your website https://ztd.bardou.online/adm
I offer mutually beneficial cooperation https://ztd.bardou.online/adm
Cool website. There is a suggestion https://ztd.bardou.online/adm
I really liked your site. Do you mind https://ztd.bardou.online/adm
Content for your website https://ztd.bardou.online/adm
Web Development Wizards https://ztd.bardou.online/adm
Can provide a link mass to your website https://ztd.bardou.online/adm
Free analysis of your website https://ztd.bardou.online/adm
SEO Optimizers Team https://ztd.bardou.online/adm
I offer mutually beneficial cooperation https://ztd.bardou.online/adm
Hi i am kavin, its my first time to commenting
anywhere, when i read this piece of writing i thought i could also
create comment due to this sensible paragraph.
Content for your website http://myngirls.online/
Web Development Wizards http://myngirls.online/
Can provide a link mass to your website http://myngirls.online/
Your site’s position in the search results http://myngirls.online/
Free analysis of your website http://myngirls.online/
SEO Optimizers Team http://myngirls.online/
I offer mutually beneficial cooperation http://myngirls.online/
Content for your website http://fertus.shop/info/
Web Development Wizards http://fertus.shop/info/
Can provide a link mass to your website http://fertus.shop/info/
Your site’s position in the search results http://fertus.shop/info/
Free analysis of your website http://fertus.shop/info/
I offer mutually beneficial cooperation http://fertus.shop/info/
I really liked your site. Do you mind http://fertus.shop/info/
Here’s what I can offer for the near future http://fertus.shop/info/
You will definitely like it http://fertus.shop/info/
The best prices from the best providers http://fertus.shop/info/
Additional earnings on your website http://fertus.shop/info/
Analytics of your website http://fertus.shop/info/
I would like to post an article http://fertus.shop/info/
How to contact the administrator on this issue http://fertus.shop/info/
Shall we exchange links? My website http://fertus.shop/info/
We offer cooperation on SEO optimization http://fertus.shop/info/
Web Development Wizards http://fertus.shop/info/
Can provide a link mass to your website http://fertus.shop/info/
Content for your website http://fertus.shop/info/
Надежная перетяжка мебели в Минске по доступной цене
перетяжка мебели в минске недорого https://obivka-divana.ru/ .
?Bonos exclusivos! Bono casino sin deposito
bono sin deposito casino peru casino online bono por registro sin deposito .
Почему стоит выбрать рулонный газон для своего сада
рулонный газон https://rulonnyygazon177.ru/ .
Атмосфера здоровья и комфорта
5. Дом из бруса 9х12: гармония с природой
дом брус 9х12 https://domizbrusa9x12spb.ru/ .
Juegos de casino en linea en Peru para todos los gustos
mejor casino online peru online casino games peru .